Definition: What does GMP mean?
GMP is the abbreviation for Good Manufacturing Practice. GMP refers to guidelines for quality assurance of production processes and environments - primarily in the pharmaceutical, food, animal feed and cosmetics industries.
GMP was introduced by the U.S. Food and Drug Administration (FDA) in 1962 and legally implemented in Germany on November 3, 2006 as part of the German Drug and Active Ingredient Manufacturing Ordinance (AMWHV).
All guidelines are described in detail in the EU GMP guidelines.
Contents of quality management according to GMP
A Good Manufacturing Practice cannot be defined arbitrarily by each company. Rather, there are strict regulations as to which measures are to be implemented and how. Accordingly, GMP defines fixed areas of activity for quality management.
Document management
Good Manufacturing Practice stipulates that the versions of specification documents must be controlled and have a defined life cycle - i.e. they cannot continue to run unrevised for all eternity. Records must be created and archived in accordance with documentation and data integrity principles. In addition, a digital audit trail is required in the computer systems.
Deviation management
Deviations happen and are often not planned. In accordance with GMP, such deviations and unapproved changes must be thoroughly documented, evaluated and checked by quality assurance.
Change management
In contrast to deviation management, change management deals with planned changes. These must be justified, approved and documented before implementation.
Qualification of equipment
Good manufacturing practice also depends to a large extent on the equipment used. Accordingly, good manufacturing practice stipulates that the suitability and reliability of systems, equipment and rooms must be regularly checked and verified.
Validation
Validation is primarily about regularly checking the processes and methods used. Do they deliver reliable, reproducible results? Do the results correspond to the selected product?
Employee training
GMP stipulates that employees must receive regular training, especially before starting work.
Risk management
Risk management is also an essential part of GMP. Production risks should therefore be systematically assessed and controlled.
Internal audits
What about process quality? Are all guidelines being adhered to? Are there any problems with document management? These and other questions must be answered in regular self-audits.
Complaints system
What happens in the event of complaints? The complaints handling department should always have an answer to this question - in advance and for every conceivable case.
Summary:
- document control: versioning, archiving and a digital audit trail are mandatory
- deviations and changes must be documented, evaluated and approved
- technology and processes must be reviewed regularly
- training and risk management: train employees, identify and manage risks
- audits and complaints: Carry out internal audits, process complaints in a structured manner
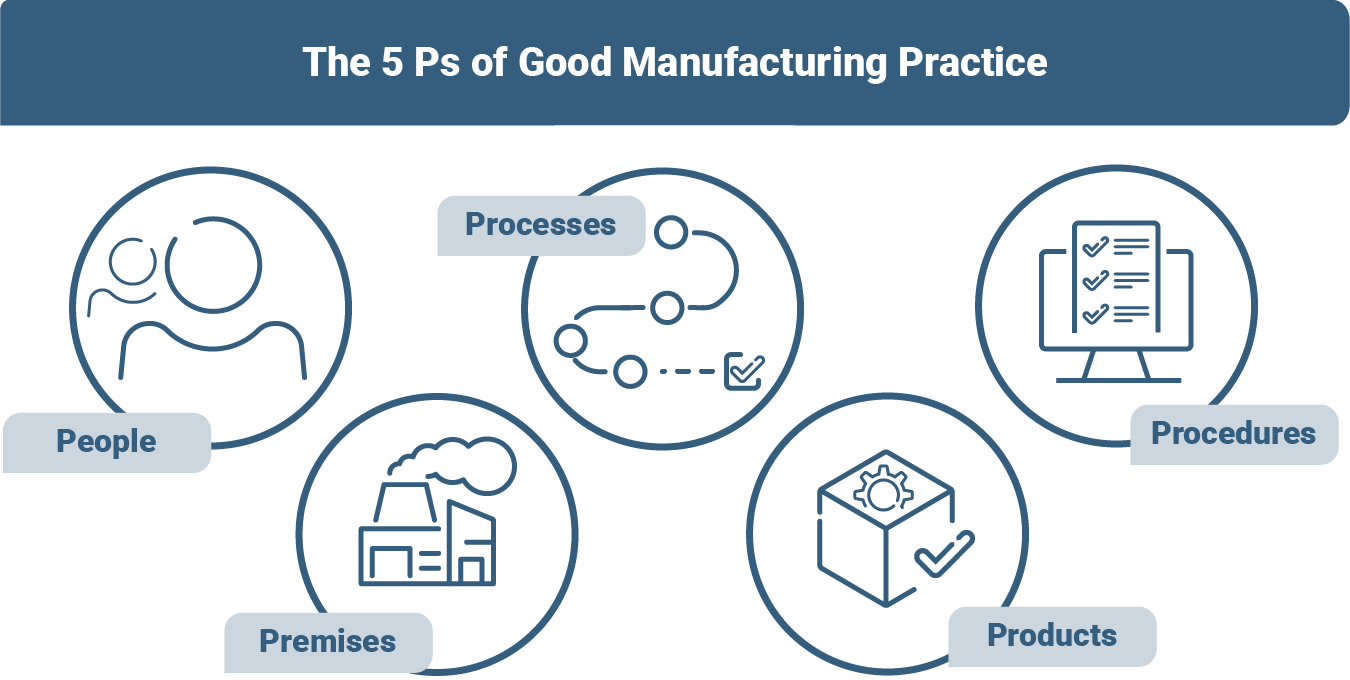
The 5 Ps of Good Manufacturing Practice
Over the years, five key components - the 5 Ps of Good Manufacturing Practice - have been established to help keep the requirements of GMP in view and ultimately comply with them. The 5Ps are:
- People: You need well-trained, skilled and qualified staff.
- Premises: You must have suitable, clean and controlled premises and facilities.
- Processes: Establish validated, standardized and controlled manufacturing processes.
- Products: Your products must be safe, effective and of consistent quality.
- Procedures: Use clear, documented work instructions and SOPs and implement them consistently.
The advantages of GMP
There are a lot of regulations and guidelines behind the rather harmless-sounding Good Manufacturing Practice. However, these, in turn, do have advantages for your company.
In order to comply with the guidelines, you will inevitably have to adapt your internal processes. Sooner or later, this will result in optimized, more efficient processes.
Since GMP is a quality management tool, you will benefit from higher quality standards, consistently high product quality and a greater awareness of quality in your company if you implement it consistently.
Of course, the user of your products is particularly important with GMP. With GMP guidelines, you can ensure that cross-contamination, contamination and mislabeling are prevented. This protects consumers from inconvenience or health risks.
GMP also helps you to comply with documentation requirements and legal regulations - and this protects you from legal consequences.
Summary:
- GMP forces process optimization and increases operational efficiency.
- GMP strengthens product quality and quality awareness within the company.
- GMP protects users from risks and companies from legal consequences.
Objectives of GMP
Let's get back to the point. Good Manufacturing Practice aims to
- ensure high, consistent product quality
- meet regulatory marketing requirements
- protect consumers from the dangers of defective products
Best practice for your production
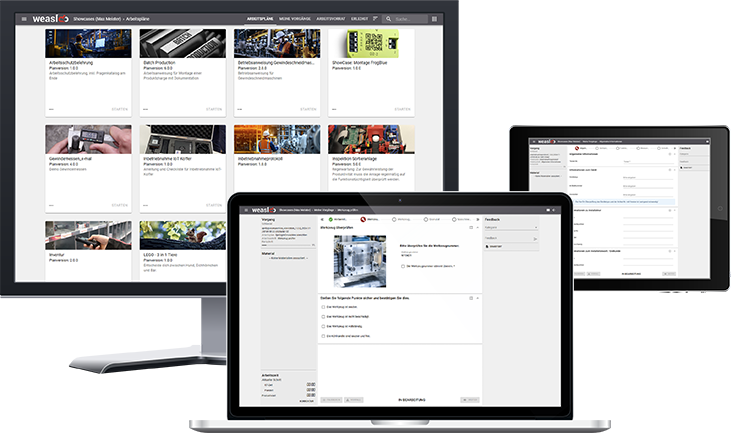
The easiest way to achieve the highest product quality, efficient employee qualification and transparent documentation is through a digital system. Our worker guidance system weasl comes with an extensive tool set to provide you and your employees with optimum support.
Experience it for yourself - in our free showcase environment.